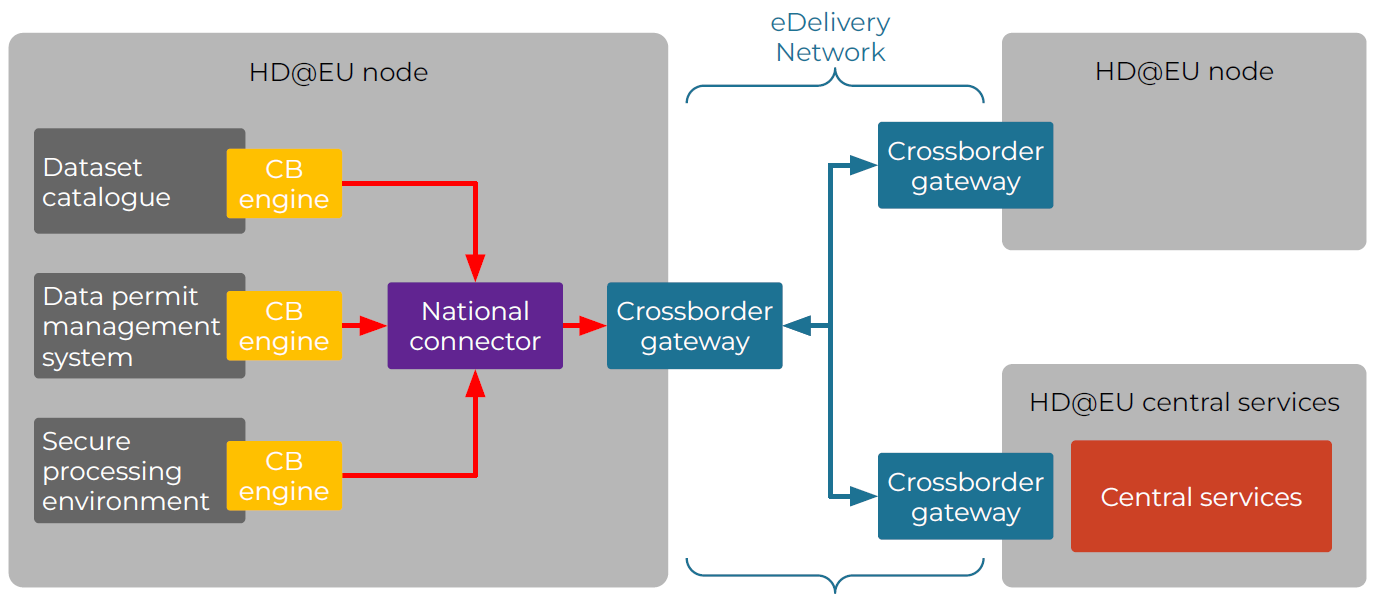
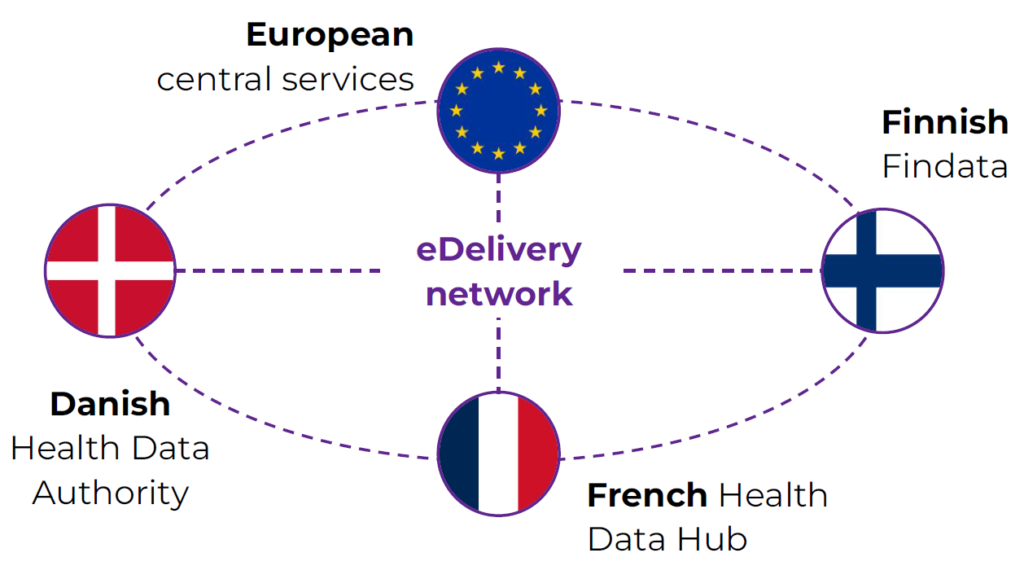
Laut einer Umfrage von BEUC (Europäischer Verbraucherverband) lehnen viele Bürgerinnen und Bürger der Europäischen Union es ab, Ihre Gesundheitsdaten mit Ärzten im Ausland zu teilen. Die repräsentative Umfrage in acht EU-Ländern, darunter Deutschland, zeigt deutliche Unterschiede dahingehend auf, welche Daten welchen Institutionen preisgegeben würden.
- Die Mehrheit (61 Prozent) der Befragten habe beispielsweise kein Problem damit, allgemeine Informationen zum Gesundheitszustand, unter anderem zu Allergien und Krankheiten, zu teilen.
- Knapp 70 Prozent wollen laut Umfrage aber keine Auskunft über Gewohnheiten wie Ernährung, Bewegung und Drogenkonsum geben. Genetische Daten oder Angaben zur sexuellen Gesundheit will kaum jemand teilen.
- Die Bereitschaft, Daten zu teilen, hänge auch stark von Vertrauen ab: Ihrem Hausarzt würden 88 Prozent der Befragten ihre Daten anvertrauen, ihrer Versicherung nur 8 Prozent.
Hintergrund der Arbeit ist der Verordungsentwurf der EU-Kommission für den Europäischen Gesundheitsdatenraum (European Health Data Space – EHDS)“: Patienten könnten ihre Krankengeschichte, Testergebnisse oder Verschreibungen dann mit Krankenhäusern und Ärzten in der gesamten EU teilen. Europaparlament und die EU-Staaten müssen noch einen Kompromiss aushandeln.
Ein Arzt in Frankreich könne dann etwa die Krankengeschichte eines Portugiesen einsehen, der in Paris krank wird, und die richtigen Medikamente verschreiben, sagte EU-Gesundheitskommissarin Stella Kyriakides vor genau einem Jahr. Unnötige Untersuchungen würden überflüssig. Das zweite Ziel des Vorschlags ist, dass Forscher, Industrie und öffentliche Institutionen das Potenzial der Daten nutzen können.
BEUC-Studie: Download des Ergebnisberichts
Executive summary: With the European Union institutions currently working to create a European health data space, which would allow the access and sharing of people’s health data across the EU, nine consumer organisations from eight EU countries have surveyed people to get a better insight on what they think about sharing their health data.
This survey sheds light on consumer attitudes and unveils what type of data they are willing to share, with whom and for what purpose (for provision of healthcare, for scientific research, or for public health reasons).
- Our survey reveals that consumers are generally cautious about sharing their health data through online health platforms and want to decide for themselves what data they share and with whom.
- Health data is rightly regarded as both extremely sensitive and of great value by all kinds of entities, from public health authorities and businesses to people themselves.
- As a result, it is crucial that EU legislators respect consumers’ interests, and our evidence-based recommendations should provide food for thought at this critical juncture.